Droplets on demand: a simpler route to microfluidic mastery
GA, UNITED STATES, June 23, 2025 /EINPresswire.com/ -- Precision in droplet formation is key to advancing lab-on-a-chip technologies. Now, researchers have unveiled a user-friendly method that generates individual droplets on demand with pinpoint accuracy. Using a modified microfluidic T-junction, the team combined fluid mechanics with experimental validation to predict the pressure thresholds needed to start and stop droplet formation. Their model was tested under various configurations and successfully adapted in real time through visual feedback. This innovation opens up a path to smarter, more flexible droplet-based systems, making it easier to design programmable microfluidic tools for chemical analysis, diagnostics, and biological research.
Microfluidic droplets serve as miniature laboratories, enabling precise control of chemical reactions, cell assays, and molecular diagnostics. Traditionally, these droplets are created passively using fixed flow rates, but this approach lacks flexibility and fails when real-time manipulation of individual droplets is needed. Active methods offer more control but often require bulky external systems. T-junction geometries provide a compact alternative, yet their dependence on trial-and-error tuning has limited real-world applications. These challenges underscore a growing demand for a simple, predictive method that enables reliable, on-demand droplet generation with minimal complexity. Due to these challenges, there is a growing need to develop reliable, easy-to-integrate methods for generating droplets on demand.
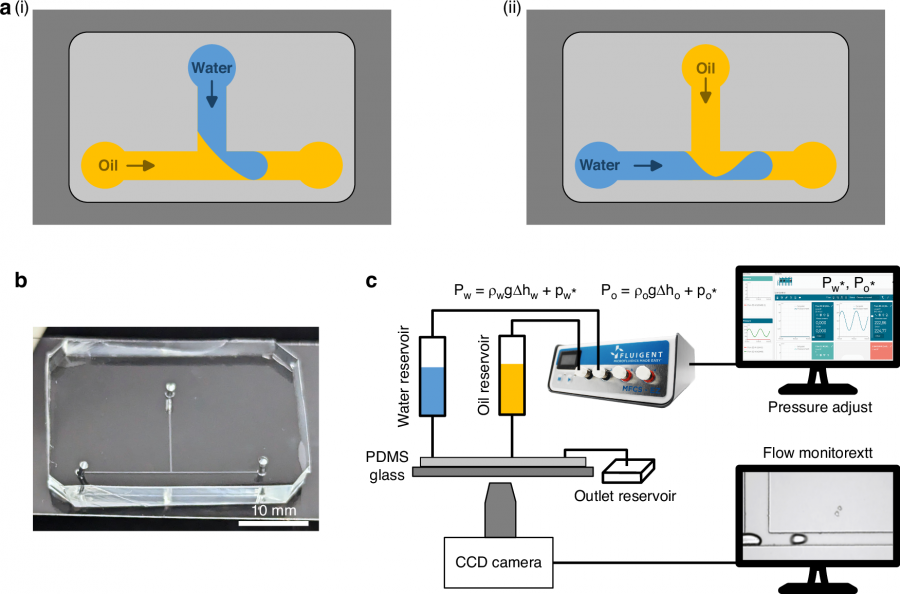
In a study published May 19, 2025, in Microsystems & Nanoengineering, a collaborative team from the University of Glasgow and the Changchun Institute of Optics introduced a new way to generate droplets at a microfluidic T-junction. By inverting the traditional layout—feeding oil through the side channel instead of water—they achieved precise, pressure-driven control over droplet formation. The team also developed and validated a theoretical model to predict the pressures required for switching droplet creation on and off, offering a fast and practical solution for real-time microfluidic control.
The innovation lies in a subtle yet powerful redesign of the classic T-junction. Instead of letting water shear off into oil, the new setup allows oil to intercept the water stream from the side—essentially slicing off droplets like a guillotine under pressure. This inversion, combined with a model grounded in capillary physics, enables researchers to accurately forecast when droplets will form or stop, based on inlet pressures and channel geometry. The model's predictions were validated through experiments involving different device configurations and surfactant-modified oils.
To tackle real-world inconsistencies, such as surface tension variations, the researchers introduced a visual feedback method: tracking droplet generation frequency to fine-tune pressure settings in real time. This dynamic tuning expands operational flexibility without requiring complex instrumentation. Tests showed the system worked reliably across designs, with control precise enough to generate single droplets at timed intervals—essential for applications like cell encapsulation or timed reagent delivery. The team’s approach transforms droplet control from a guesswork process into an engineering discipline.
"Our aim was to make droplet microfluidics smarter, not harder," said Professor Huabing Yin, the study's senior author. "We've shown that with a straightforward change in geometry and a bit of physics, we can turn a previously unpredictable system into one you can program and control. That's a game-changer for labs looking to automate and scale up."
The implications are far-reaching. From biomedical diagnostics to synthetic biology, any field requiring precise fluid handling can benefit from this model-guided droplet system. Its low barrier to implementation—no external fields or complex electronics—makes it especially attractive for portable or point-of-care devices. With customizable droplet size and timing, researchers can now design microfluidic systems tailored to their specific tasks. As labs continue to miniaturize and automate, this innovation could become a cornerstone of next-generation lab-on-a-chip platforms.
References
DOI
10.1038/s41378-025-00950-2
Original Source URL
https://doi.org/10.1038/s41378-025-00950-2
Funding information
The NERC (NE/S008721/1), Innovate UK project (104984) and EPSRC IAA (EP/R511705/1) for financial support. William Mills also thanks EPSRC lifETIME CDT (EP/S02347X/1) for the scholarship.
Lucy Wang
BioDesign Research
email us here
Distribution channels: Science, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release