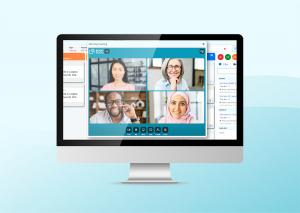
GxP Inspection Readiness Software, Ready Room, Now Includes Video Conferencing
Ready Room, a SaaS platform used by life science and medtech companies to efficiently manage their GxP readiness inspections, adds secured video conferencing.
Ready Room briefings, which utilize the Whereby video platform, offer secure, high quality, browser-based audio and video with integrated screen sharing and chat. Briefings are useful for daily debriefs, preparation of interviewees, strategy sessions with remote subject-matter experts, and interviews.
Commenting on the importance of the new release of Ready Room, Peter Lacey, Chief Technology Officer, said, “Complexity equals risk, and Ready Room’s purpose is to reduce risk during an audit or inspection. With this release, we remove the complications of switching to an external video conferencing tool such as Microsoft Teams® or zoom® to collaborate with subject matter experts, vendors, contractors, and management. Now live meetings with team members are just a click away.”
Ready Room replaces an organization’s ad hoc collection of tools typically used to manage inspections, such as slide decks, email, file sharing, and meeting software, with an intuitive drag-and-drop interface, which greatly improves speed and accuracy in delivering inspection requests. Ready Room also provides unique storyboard functionality, including standard inspection questions and prompts for preparing interviewee talking points, which helps the inspection team build a “quality story” to deliver during the inspection.
Denise Lacey, Ready Room’s founder and President of parent company, Synclinical Quality Assurance, stated, “We developed Ready Room because we needed it. We knew if we could consolidate our unique tools into an integrated system—and incorporate our knowledge of inspection and audit expectations—life science teams of all kinds could build and deliver a quality story with each inspection. Our customers tell us that Ready Room ‘just works the way inspections do.’ “
Learn more about Ready Room, the cloud-based inspection readiness and management platform for GxP inspections, by visiting www.readyroom.net
About Ready Room | www.readyroom.net
Originally developed by Synclinical Quality Assurance, LLC to help its clients prepare for and manage FDA inspections and audits, Ready Room is now a fully optimized commercial SaaS platform. Customers include a variety of life-science companies and organizations such as pharmaceutical, biotech, and medical device manufacturers; contract manufacturers; research laboratories; and institutional review boards.
Ready Room Contact – Denise Lacey, dlacey@synclinical.com
Agency Contact – David Strand, dstrand@nowspeed.com
David Strand
Nowspeed
+1 508-919-5518
email us here
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.